Advanced Diagnostics & Personalised Medicine.
Imagine a world where diseases such as cancer, HIV, malaria, tuberculosis, heart failure and Ebola could be detected as simply, quickly and cheaply as pregnancy is today.
We are transforming disease detection by harnessing the mighty power of miniscule nanomaterials to make this dream a reality. These nanomaterials react with chemicals produced by diseases in the body and cause visible colour changes in urine tests, and in tests similar to home pregnancy tests.
Recognising the ubiquitous power of the mobile-connected modern world, we are co-developing a smartphone platforms which capture and record data from the tests to track the spread and treatment of infectious diseases across communities.
Our long-term goal is to democratise and personalise healthcare with these ultrasensitive, cost-effective, user-friendly and mobile connected technologies. To do this, we work with hospitals in Europe, the US and in Africa. You can read more about a selected example of projects below.
We use designer nanoparticles to help detect diseases earlier.
Image credit: Loynachan, Thomas et al. ACS Nano. 2018. Adapted under the terms of a CC-BY License.
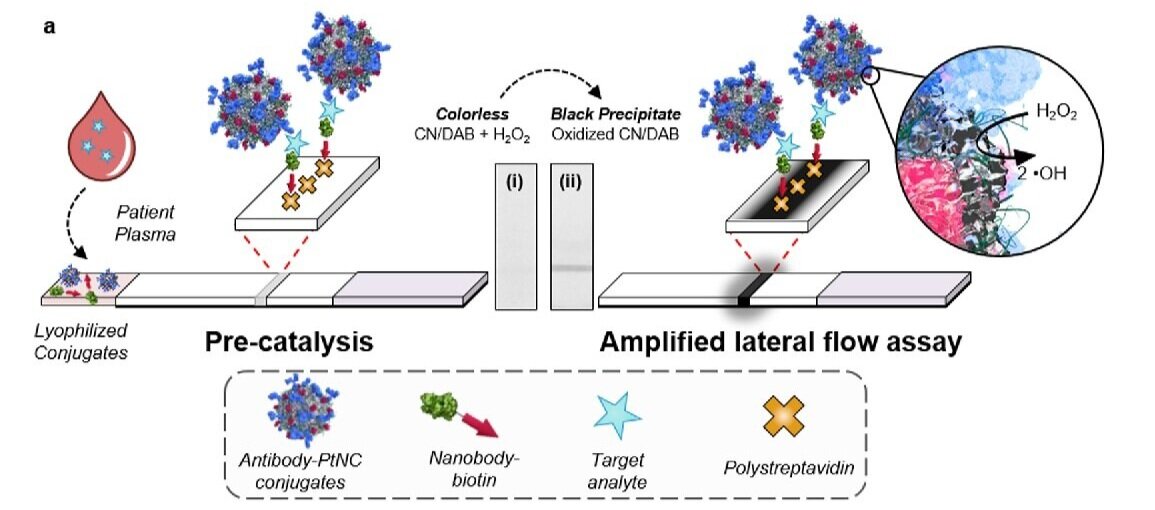
Since the early 19th Century, platinum has been recognised as a catalyst; third-party substances which increase the speed of chemical reactions. Today it is widely used inside catalytic converters to scrub nasty pollutants from vehicle exhausts.
We are channelling this catalytic capability to increase the sensitivity of paper-based lateral-flow immunoassays - the same technology employed by simple home pregnancy kits. These point-of-care diagnostic devices must provide a read-out from small sample sizes with a rapid turnaround, but traditional tests struggle to produce a clear result.
In our devices, a patient’s sample is applied to the paper. If there are proteins associated with the disease present in the sample, for example HIV, these will bind to the platinum nanocatalysts on the paper to form a complex. There is a thin strip on the paper which binds to the target proteins, trapping them - and the nanocatalyst complexes - there. After twenty minutes, the platinum nanocatalysts catalyse an oxidation reaction at the strip site, which produces a dark black pigment, clearly visible to the naked eye. In this way, the nanocatalysts amplify the signal, and produce a device which is 100 times more sensitive than traditional tests.
The image on the right shows a transmission electron microscopy image of these materials. Each dark object represents a single gold nanoparticle, coated in a fuzzy shell of platinum.
We connect data from infectious disease diagnostic tests with smartphone technology.
Smartphone devices are becoming more widespread and increasingly integral to modern life in both developed and developing countries. This ubiquity brings great potential for health services to gather data about infectious diseases at a population level. Being able to track, trace and treat the spread of an infectious disease is critical to bringing outbreaks under control. This field is called mHealth.
Together with the i-sense research centre, we have analysed the potential for impact of smartphone-based health systems on low-income countries, where health clinics in rural areas can be scarce. Click here to read the paper in Nature.
One example is a paper-based diagnostic test for Ebola which reveals the presence of related antibodies in a person’s urine. These cheap and accurate tests can be interpreted by our app using the camera on a person’s smartphone. The app interprets the results of the test and registers the patient’s geo-location from the device, allowing us to capture and record information across wide geographical areas. These data can be used during and after epidemics of infectious diseases to effectively track, trace and treat an outbreak.
We use gold nanoclusters inside the body to detect early disease.
Image credit: Loynachan et al. 2018. Reproduced under terms of CC-BY license.
Gold nanoclusters are roughly a million times smaller than a full-stop. Similar, miniscule particles of gold have been used since Antiquity for their ability to change colour with their size and chemical environment; the Romans used suspensions of gold nanoparticles to make colour-changing glass. Medicinal use gold began in the Middle Ages, and today we are collaborating with Sangeeta Bhatia’s group at MIT to detect a range of diseases including cancer.
It works like this. The gold nanoclusters are joined to particular proteins to create a relatively large ‘complex’, and these are injected into the body. In healthy people, these complexes would be too large to be broken down by the kidneys and then pass through into the urine. Diseases such as cancer and HIV produce molecules called proteases, whose job is to break down proteins. By matching the proteins on the nanocluster complex with those targeted by disease proteases, complexes injected into diseased patients get broken down by the proteases into smaller components. Once this happens, the gold nanoclusters are then small enough to pass through the kidneys and into the urine. A simple test can produce a colour change reaction of the urine to turn it blue in patients with markers of the targeted disease (see image).
We build artificial noses to sniff out disease.
Many traditional diagnostic tests rely on capturing disease-specific molecules from diseases, such as antibodies. Our Functionalized Array for Surface-Enhanced Raman Spectroscopy (FASERS) technology moves away from targeting specific disease structures and uses a surface-enhanced Raman spectroscopy method which does not require the target disease structure to be pre-labelled.
Our platform is called an artificial nose, which is a special surface that effectively sniffs out a range of different biological molecules in a sample. These molecules bind onto the platform’s surface, creating a unique chemical fingerprint, and high dimensionality data sets.
The platform has been tested by comparing the fingerprints of breast-cancer and non-cancerous cells, and has proven to be very accurate. It opens up the possibility for a powerful label-free diagnostic platform with high dimensionality.
Selected Publications.
N. Kim, M. R. Thomas, M. S. Bergholt, I. J. Pence, H. Seong, P. Charchar, N. Todorova, A. Nagelkerke, A. Belessiotis-Richards, D. J. Payne, A. Gelmi, I. Yarovsky and M. M. Stevens
“Surface enhanced Raman scattering artificial nose for high dimensionality fingerprinting”
Nature Communications. 2020. 11: 207.
C. N. Loynachan, A. P. Soleimany, J. S. Dudani, Y. Lin, A. Najer, A. Bekdemir, Q. Chen, S. N. Bhatia and M. M. Stevens
”Renal clearable catalytic gold nanoclusters for in vivo disease monitoring.”
Nature Nanotechnology. 2019. 14 (9): 883-890.
C. S. Wood, M. R. Thomas, J. Budd, T. P. Mashamba-Thompson, K. Herbst, D. Pillay, R. W. Peeling, A. M. Johnson, R. A. McKendry and M. M. Stevens
”Taking connected mobile-health diagnostics of infectious diseases to the field.”
Nature. 2019. 566 (7745): 467-474.
Y. Lin, P. Charchar, A. J. Christofferson, M. R. Thomas, N. Todorova, M. M. Mazo, Q. Chen, J. Doutch, R. Richardson, I. Yarovsky and M. M. Stevens
”Surface dynamics and ligand–core interactions of quantum sized photoluminescent gold nanoclusters.”
Journal of the American Chemical Society. 2018. 140 (51): 18217-18226.
Y. Wang, P. D. Howes, E. Kim, C. D. Spicer, M. R. Thomas, Y. Lin, S. W. Crowder, I. J. Pence and M. M. Stevens
”Duplex-specific nuclease-amplified detection of MicroRNA using compact quantum dot–DNA conjugates.”
ACS Applied Materials & Interfaces. 2018. 10 (34): 28290-28300.
A. Creamer, C. S. Wood, P. D. Howes, A. Casey, S. Cong, A. V. Marsh, R. Godin, J. Panidi, T. D. Anthopoulos, C. H. Burgess, T. Wu, Z. Fei, I. Hamilton, M. A. McLachlan, M. M. Stevens, and M. Heeney
”Post-polymerisation functionalisation of conjugated polymer backbones and its application in multi-functional emissive nanoparticles.”
Nature Communications. 2018. 9 (1): 1-11.
P. Brangel, A. Sobarzo, C. Parolo, B. S. Miller, P. D. Howes, S. Gelkop, J. J. Lutwama, J. M. Dye, R. A. McKendry, L. Lobel and M. M. Stevens
”A Serological Point-of-Care Test for the Detection of IgG Antibodies against Ebola Virus in Human Survivors.”
ACS Nano. 2018. 12 (1): 63-73.
C. N. Loynachan, M. R. Thomas, E. R. Gray, D. A. Richards, J. Kim, B. S. Miller, J. C. Brookes, S. Agarwal, V. Chudasama, R. A. McKendry and M. M. Stevens
”Platinum Nanocatalyst Amplification: Redefining the Gold Standard for Lateral Flow Immunoassays with Ultrabroad Dynamic Range”
ACS Nano. 2018. 12(1): 279–288.