Engineering & Exploring the Bio-Material Interface.
Our bodies contain around 30 trillion cells. Smaller than the eye can see, these cells make up the tissues of our bones, flesh and organs. Sometimes we may want to introduce non-living matter into the living human body, such as a medical implant. From gold teeth to wooden legs, non-living materials have a long history of interfacing with the human body. But what happens to the cells at the interface between living and non-living matter? And what if we could control their interaction?
Our work involves influencing the behaviour of cells at the interface of living and non-living matter by tweaking the surface chemistry and texture of materials such as nanoparticles and nanoneedles. With such minuscule subjects, these experiments frequently push the limits of what existing technologies and characterisation methods are capable of.
So we decided to invent some of our own. We develop cutting-edge tools and techniques for studying cells, tissues and biomaterials.
Scroll down to read more about some examples of our projects.
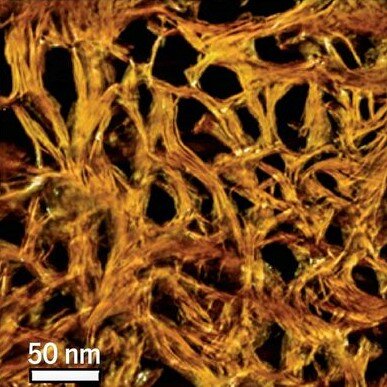
We use nano-scale surface texture to understand cell behaviour.
Image credit: Charlotte Lee-Reeves.
Living cells are inherently sensitive to the surface texture and chemistry of their local environment.
This is a microscope image of cells sitting on top of our nanoneedle engineered surfaces. Some types of cell elongate along the nanoneedle grid, giving these patterns of extremely stretched cells. We can observe differences in cell behaviour such as shape and gene expression by changing the shape, size, spacing and chemical coating of the nanoneedle grid. Click here to read the paper in Advanced Materials.
Understanding how cells interact with this extreme type of surface gives us insight into the fundamental mechanisms that allow cells to sense and respond to their environment. Once we understand cell behaviour, we can start engineering surfaces to influence it to our advantage. Click here to read the paper in ACS Nano.
We’ve even shown, in collaboration with HMRI, that nanoneedles can deliver DNA and RNA directly into cells, allowing for cells in selected areas of tissue to be reprogrammed to, for example, induce the formation of new blood vessels. Click here to read the paper in Nature Materials.
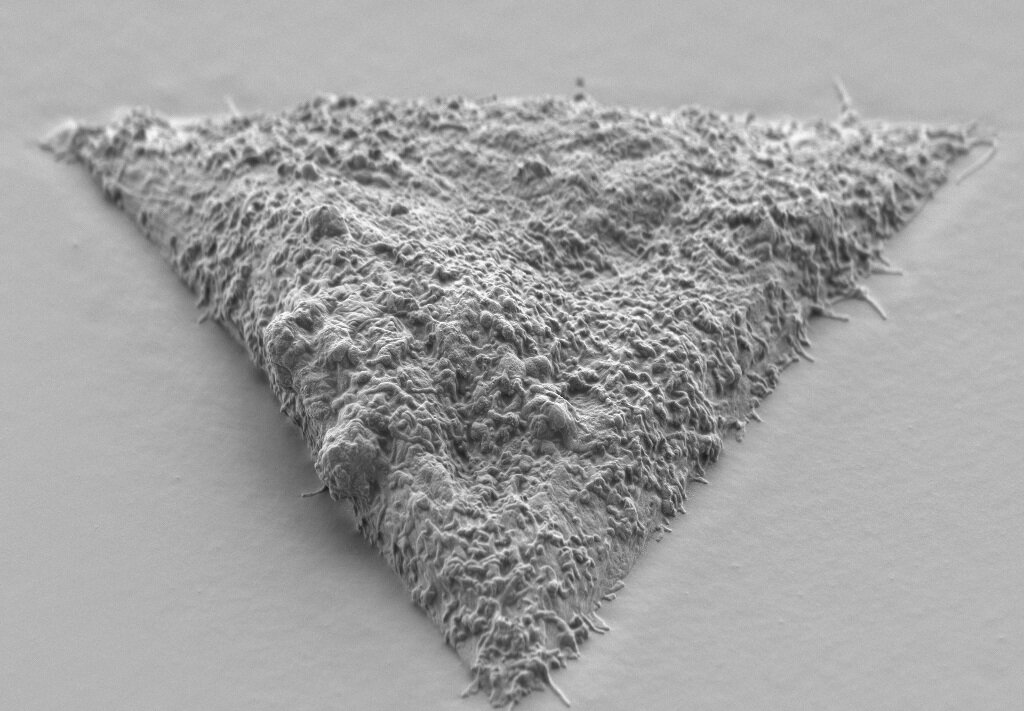
We manipulate the shape of cells to study how they work.
The shape of cells is critically important for their biological functions. In particular, a cell’s shape can affect their signalling, the mechanism which helps them to adapt quickly to changes in tissue mechanics.
We use materials surface engineering to encourage cells to adopt different geometries, such as circles, squares and triangles. By using innovative characterisation and microscopy methods such as focused ion beam scanning electron microscopy (FIB-SEM), we study the mechanisms responsible for cell signalling of these cells in extreme contortions.
These techniques allow us to see cell-material interactions with nanoscale precision. We use beams of charged particles called ions to slice through the interface of soft cells on hard surfaces such as silicon, and scanning electron microscopy to study the results.
We are innovating nanoparticle analysis with our SPARTA™ technology.
SPARTA™ (Single Particle Automated Raman Trapping Analysis) is an instrument we invented and developed to analyse and characterise single nanoparticles.
It might seem strange that mere light has the power to move and manipulate solid matter. But in the realm of the nanoscale, new rules take over, and concentrated laser beams are able to do just that.
Once the nanoparticle in question is successfully trapped, SPARTA™ uses laser light to decode information from it such as chemical composition, particle size and surface chemical reactions. Unlike other competing technologies, it can all be done in an automated, high-throughput, non-destructive and label-free manner.
This technology is ready for commercialisation, and will be a game-changer to our academic and industrial partners in drug development, drug delivery and gene therapy research.
We make breakthroughs in discovering the structure of bone.
It has long been known that the structure of our bones is hierarchical; meaning that the largest structural components are made from smaller structures, which themselves are made from smaller structures and so on, right down to the atomic scale. This ingenious structuring gives bone its requisite stiffness and toughness, properties usually considered mutually exclusive.
The material of bone is in itself a composite material; a mixture of two different materials, in this case mineral and collagen. However, it was previously not known exactly how these two components structured themselves in order to build the hierarchical make-up of bone.
We used a mixture of electron diffraction, scanning transmission electron microscopy (STEM) and 3D reconstruction to visualise the nanoscale structure of bone in 3D. Our collaborative work with York University demonstrates previously unknown assemblies of mineral and collagen components, giving us greater insight than ever before about this most fundamental material in the human body.
Selected Publications.
H. Seong, S. G. Higgins, J. Penders, J. P. K. Armstrong, S. W. Crowder, A. C. Moore, J. E. Sero, M. Becce and M. M. Stevens
”Size-Tunable Nanoneedle Arrays for Influencing Stem Cell Morphology, Gene Expression and Nuclear Membrane Curvature.”
ACS Nano. 2020. 15(5): 5371–5381.
S. G. Higgins, M. Becce, A. Belessiotis‐Richards, H. Seong, J. E. Sero and M. M. Stevens
”High‐Aspect‐Ratio Nanostructured Surfaces as Biological Metamaterials.”
Advanced Materials. 2020. 32: 1903862.
A. Belessiotis-Richards, S. G. Higgins, B. Butterworth, M. M. Stevens and A. Alexander-Katz
”Single-Nanometer Changes in Nanopore Geometry Influence Curvature, Local Properties, and Protein Localization in Membrane Simulations.”
Nano Letters. 2019. 19 (7): 4770-4778.
C. S. Hansel, S. W. Crowder, S. Cooper, S. Gopal, M. João Pardelha da Cruz, L. de Oliveira Martins, D. Keller, S. Rothery, M. Becce, A. E. G. Cass, C. Bakal, C. Chiappini and M. M. Stevens
”Nanoneedle-mediated stimulation of cell mechanotransduction machinery.”
ACS Nano. 2019. 13 (3): 2913-2926.
S. Gopal, C. Chiappini, J. Penders, V. Leonardo, H. Seong, S. Rothery, Y. Korchev, A. Shevchuk and M. M. Stevens
”Porous silicon nanoneedles modulate endocytosis to deliver biological payloads.”
Advanced Materials. 2019. 31 (12): 1806788.
S. Gopal, C. Chiappini, J. P. K. Armstrong, Q. Chen, A. Serio, C.‐C. Hsu, C. Meinert, T. J. Klein, D. W. Hutmacher, S. Rothery and M. M. Stevens
”Immunogold FIB‐SEM: Combining Volumetric Ultrastructure Visualization with 3D Biomolecular Analysis to Dissect Cell–Environment Interactions.”
Advanced Materials. 2019. 31: 1900488.
N. Reznikov, M. Bilton, L. Lari, M. M. Stevens and R. Kröger
”Fractal-like hierarchical organization of bone begins at the nanoscale”
Science. 2018. 360 (6388): eaao2189.
T. C. von Erlach, S. Bertazzo, M. A. Wozniak, C.-M. Horejs, S. A. Maynard, S. Attwood, B. K. Robinson, H. Autefage, C. Kallepitis, A. Del Rio Hernandez, C. S. Chen, S. Goldoni, and M. M. Stevens
“Cell geometry dependent changes in plasma membrane order direct stem cell signalling and fate.”
Nature Materials. 2018. 17 (3): 237.
J. Penders, I. J. Pence, C. C. Horgan, M. S. Bergholt, C. S. Wood, A. Najer, U. Kauscher, A. Nagelkerke and M. M. Stevens
”Single particle automated raman trapping analysis.”
Nature Communications. 2018. 9 (1): 1-11.
C. Kallepitis, M. S. Bergholt, M. M. Mazo, V. Leonardo, S. C. Skaalure, S. A. Maynard and M. M. Stevens
”Quantitative volumetric Raman imaging of three dimensional cell cultures.”
Nature Communications. 2017. 8: 14843.
N. Reznikov, J. A. M. Steele, P. Fratzl and M. M. Stevens
"A materials science vision of extracellular matrix mineralization."
Nature Reviews Materials. 2016. 1: 16041.
C. Chiappini, E. De Rosa, J. O. Martinez, X. Liu, J. Steele, M. M. Stevens and E. Tasciotti
“Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization.”
Nature Materials. 2015. 14 (5): 532-539.